There is a substantial and rapidly expanding market opportunity, with an estimated 40 million patients currently eligible for biologic therapy across asthma, chronic obstructive pulmonary disease (COPD), and chronic rhinosinusitis with nasal polyps (CRSwNP). These three indications together represent the leading chronic inflammatory airway diseases, each contributing significantly to healthcare expenditure, lost productivity, and unmet clinical need.
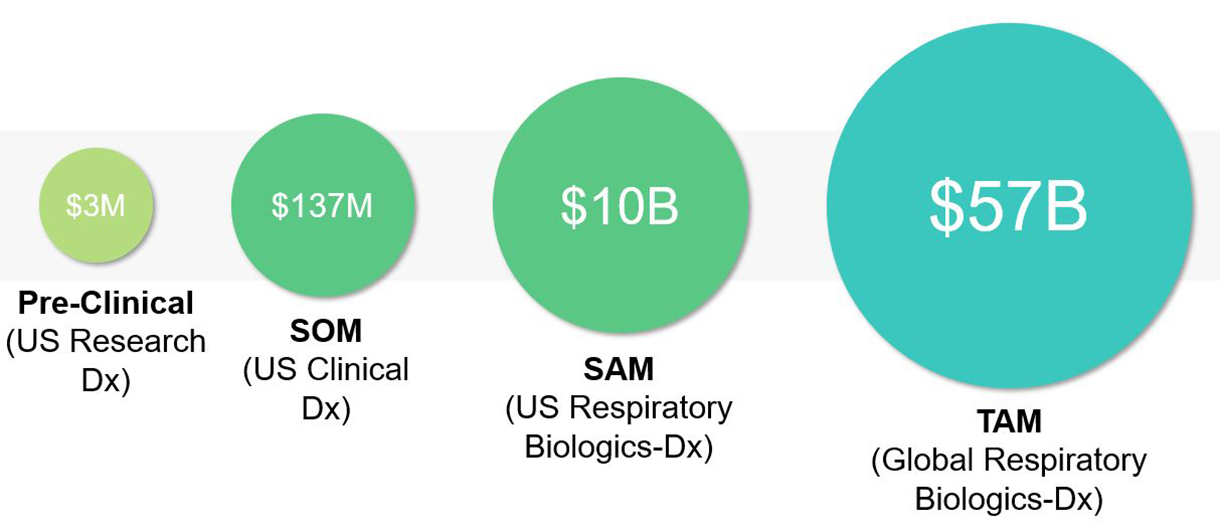
Within this landscape, the Serviceable Addressable Market (SAM) is estimated at $10 billion for U.S. respiratory biologics, reflecting the portion of the market currently accessible through existing infrastructure, payer coverage, and prescriber adoption. The Total Addressable Market (TAM), encompassing the global biologics market across key regions including the United States, EU5 (UK,FR,ES,IT,DE), China, and Japan, is projected to exceed $57 billion.
Importantly, the market continues to grow as approximately 1.5 million new patients become eligible for biologic therapy each year. This represents an additional $2.1 billion in annual revenue potential, underscoring both the clinical and commercial scale of opportunity. As payer systems increasingly favor biomarker-guided prescribing to improve cost-effectiveness, diagnostic platforms capable of identifying likely responders will occupy a pivotal position in the evolving respiratory therapeutics ecosystem.
Nasal sampling offers a fundamentally different approach to existing solutions: it is rapid, minimally invasive, and already widely accepted by patients. The infrastructure and public familiarity established during the COVID-19 pandemic have demonstrated its scalability.
 By enabling earlier detection, patient stratification for targeted therapies, and population-level monitoring, nasal-based diagnostics have the potential to reduce healthcare costs, improve clinical decision-making, and expand access to high-quality respiratory care.
By enabling earlier detection, patient stratification for targeted therapies, and population-level monitoring, nasal-based diagnostics have the potential to reduce healthcare costs, improve clinical decision-making, and expand access to high-quality respiratory care.
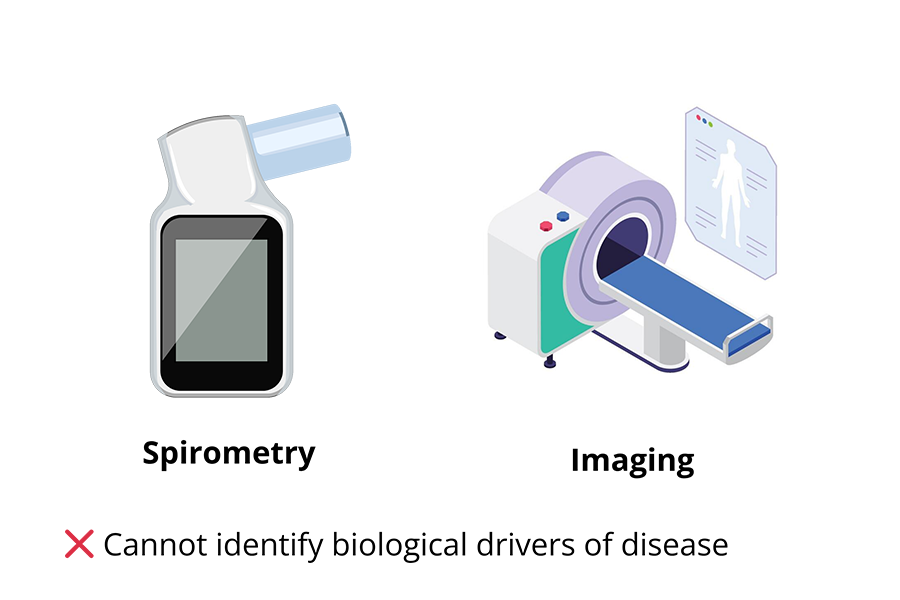
Conventional tools like spirometry and imaging detect structural or functional changes in the lungs but often miss the underlying biological cause of disease.
Diag-Nose.io’s biology-driven approach provides deeper insight by analysing local molecular signals from the airway. This allows for:
- Earlier detection of inflammation and tissue damage.
- Better understanding of disease mechanisms and progression.
- More precise monitoring of patient response over time.
- Proactive care, enabling clinicians to adjust treatment before symptoms worsen.
- Faster drug discovery, by identifying active biological pathways in real patients.
- Improved clinical trials, through smarter patient selection and stratification.
 Compared with other biology-focused platforms, Diag-Nose.io’s competitive advantage lies in execution within respiratory medicine.
Compared with other biology-focused platforms, Diag-Nose.io’s competitive advantage lies in execution within respiratory medicine.
Its biology-driven approach analyses local molecular signals from the airway; the most direct source of respiratory biology, to uncover insights that blood biomarkers often miss.
Key strengths include:
- Proprietary, minimally invasive nasal sampling method capturing true airway biology.
- Exclusive IP across nasal sampling, chemistry, biomarker combinations and quality control measures.
- Improved sensitivity and specificity for airway inflammation compared with blood-based biomarkers which are subject to interference from systemic factors and comorbidities.
- Proven expertise in asthma, COPD, and sinus disease and strong clinical partnerships supporting defensibility and scale.
Value Proposition
RhinoMAP aims to deliver precision and efficiency across the respiratory care continuum by integrating nasal biomarker sampling, advanced proteomics, and AI-driven analytics. It aims to translate complex airway biology into actionable insights that clinicians can use at the point of care.
- For clinicians (ENTs, pulmonologists, allergists), RhinoMAP will help identify the right therapy faster, track disease activity objectively, and reduce the risks of ineffective or unnecessary biologic use.
- For practice managers and hospital administrators, it will improve workflow efficiency, increases diagnostic capacity, and elevates institutional visibility as a precision medicine leader.
- For payers, it will help reduce biologic waste, lowers overall costs, and strengthens the evidence base for value-based reimbursement.
- For patients, it will offer faster symptom relief, fewer treatment failures, and greater confidence in their care plan.
- For pharma partners, it will enhance trial stratification, expands biologic eligibility, and supports drug lifecycle management.
Together, these benefits advance a shared goal: precision respiratory care that is clinically effective, economically sustainable, and patient-centered.
References
- El-Deiry, W. S. et al (2019), The current state of molecular testing in the treatment of patients with solid tumors, CA: a cancer journal for clinicians, 69(4), 305–343. https://doi.org/10.3322/caac.21560
- Boers, E. et al (2023), Global Burden of Chronic Obstructive Pulmonary Disease Through 2050. JAMA network open, 6(12), e2346598. https://doi.org/10.1001/jamanetworkopen.2023.46598
- Yuan, L. et al (2025), Global, regional, national burden of asthma from 1990 to 2021, with projections of incidence to 2050: a systematic analysis of the global burden of disease study 2021, eClinicalMedicine, 80; 103051, doi: 10.1016/j.eclinm.2024.103051
- Min, H. K. et al (2025), Global Incidence and Prevalence of Chronic Rhinosinusitis: A Systematic Review. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology, 55(1), 52–66. https://doi.org/10.1111/cea.14592
- Diag-Nose (2025), Internal Market Research
- GBD Chronic Respiratory Disease Collaborators (2020). Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. Respiratory medicine, 8(6), 585–596. https://doi.org/10.1016/S2213-2600(20)30105-3
- May, S. M., & Li, J. T. (2015). Burden of chronic obstructive pulmonary disease: healthcare costs and beyond. Allergy and asthma proceedings, 36(1), 4–10. https://doi.org/10.2500/aap.2015.36.3812
- McGregor, M. et al (2018), Role of Biologics in Asthma, American Journal of Respiratory and Critical Care Medicine, 199(4), https://doi.org/10.1164/rccm.201810-1944CI
- Lanario, J. W. et al (2022), "Life-changing": the experience of super-responders to biologics in severe asthma. BMC pulmonary medicine, 22(1), 445. https://doi.org/10.1186/s12890-022-02241-2
- Ernst, D (2024), Mepolizumab is an interleukin-5 antagonist monoclonal antibody, Medical Professionals Reference, Mepolizumab Under Review for Eosinophilic COPD - MPR
- Georgakopoulou, V.E., Lempesis, I.G., Sklapani, P., Trakas, N., & Spandidos, D.A. (2024), Precision medicine for respiratory diseases: A current viewpoint. Medicine International, 4, 31. https://doi.org/10.3892/mi.2024.155
- Varricchi, G. et al (2022), Biologics and airway remodeling in severe asthma, Allergy, 77(12);3538-3552, https://doi.org/10.1111/all.15473
- Towards Healthcare (2025), Asthma And COPD Drugs Market Size, Trends & Companies Strategies, Towards Healthcare; Therapeutic Area, Asthma And COPD Drugs Market Soars USD 66.92 Bn by 2034
- Braunstahl G. J. (2007). The united airways concept: from bench to bedside. Monaldi archives for chest disease, 67(2), 95–101. https://doi.org/10.4081/monaldi.2007.496
- Braunstahl G. J. (2009). United airways concept: what does it teach us about systemic inflammation in airways disease?. Proceedings of the American Thoracic Society, 6(8), 652–654. https://doi.org/10.1513/pats.200906-052DP
- Grossman, J. (1997), One Airway, One Disease, Chest, 111(2); 11S - 16S, 10.1378/chest.111.2_Supplement.11S
- Bousquet, J. et al. (2025), From “one airway, one disease” to “one airway, many diseases”, Journal of Allergy and Clinical Immunology, 156(1); 198 - 199, doi: 10.1016/j.jaci.2025.03.022
- Feng CH, et al (2012), The united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitis, American Journal of Rhinology & Allergy, 26(3):187-190. doi: 10.2500/ajra.2012.26.3762
- Mullol, J. et al (2022), Management of United Airway Disease Focused on Patients With Asthma and Chronic Rhinosinusitis With Nasal Polyps: A Systematic Review, The Journal of Allergy and Clinical Immunology: In Practice, 10(9);2438-2447.e9, https://doi.org/10.1016/j.jaip.2022.04.039
- Gern, J. E., Vrtis, R., Grindle, K. A., Swenson, C., & Busse, W. W. (2000). Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. American journal of respiratory and critical care medicine, 162(6), 2226–2231. https://doi.org/10.1164/ajrccm.162.6.2003019
- Liew, K. Y., Koh, S. K., Hooi, S. L., Ng, M. K. L., Chee, H. Y., Harith, H. H., Israf, D. A., & Tham, C. L. (2022). Rhinovirus-Induced Cytokine Alterations With Potential Implications in Asthma Exacerbations: A Systematic Review and Meta-Analysis. Frontiers in immunology, 13, 782936. https://doi.org/10.3389/fimmu.2022.782936
- Hansel, T. et al (2017), A Comprehensive Evaluation of Nasal and Bronchial Cytokines and Chemokines Following Experimental Rhinovirus Infection in Allergic Asthma: Increased Interferons (IFN-γ and IFN-λ) and Type 2 Inflammation (IL-5 and IL-13). EBioMedicine, 19, 128–138. https://doi.org/10.1016/j.ebiom.2017.03.033
- Roberts, N. et al (2018), Comparison of paired human nasal and bronchial airway epithelial cell responses to rhinovirus infection and IL-13 treatment, Clinical and translational medicine, 7(1);13. https://doi.org/10.1186/s40169-018-0189-2
- Li, X. et al (2021), The role of IL-8 in the chronic airway inflammation and its research progress, Journal of Clinical Otorhinolaryngology Head and Neck Surgery, 35(12):1144-1148, https://doi.org/10.13201/j.issn.2096-7993.2021.12.020
- Diag-Nose Medical (2025). ABEL Microsampler® Registered on the ARTG – Nasal Liquid Biopsy device media releasenewshub-website-data.s3.ap-southeast 2.amazonaws.comnewshub-website-data.s3.ap-southeast-2.amazonaws.com
- Reza, M. and Ambhore, N. (2025), Inflammation in Asthma: Mechanistic Insights and the Role of Biologics in Therapeutic Frontiers, Biomedicines, 13(6), 1342; doi:https://doi.org/10.3390/biomedicines13061342
- Shaibie, N.A., Mohammad Faizal, N.D.F., Buang, F. et al. (2025) Inhaled biologics for respiratory diseases: clinical potential and emerging technologies. Drug Deliv. and Transl. Res. doi: https://doi.org/10.1007/s13346-025-01909-6
- Brusselle, G. G., & Koppelman, G. H. (2022). Biologic Therapies for Severe Asthma. The New England journal of medicine, 386(2), 157–171. https://doi.org/10.1056/NEJMra2032506
- Dierick, B. J. H. et al (2024). Reshaping respiratory care: potential advances in inhaled pharmacotherapy in asthma. Expert opinion on pharmacotherapy, 25(11), 1507–1516. https://doi.org/10.1080/14656566.2024.2389258
- Menzella F. (2023), Baseline characteristics of patients enrolled in clinical trials of biologics for severe asthma as potential predictors of outcomes. J Clin Med,12(4);1546, doi: 10.3390/jcm12041546
- Pelaia, C. et al (2021), Tezepelumab: A Potential New Biological Therapy for Severe Refractory Asthma. International journal of molecular sciences, 22(9), 4369. https://doi.org/10.3390/ijms22094369